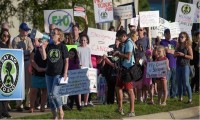-
This technology could enable time-released drug delivery
- Source: drugdu
- 405
- April 14, 2023
-
Appeals court rules abortion drug can stay on market — but limits access
- Source: drugdu
- 526
- April 14, 2023
-
Chiesi Farmaceutici acquires biopharmaceutical firm Amryt Pharma
- Source: drugdu
- 402
- April 14, 2023
-
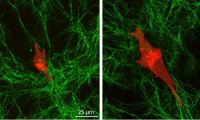
Engineers find cancer cells have remarkable ability to penetrate deep into their environment
- Source: drugdu
- 353
- April 13, 2023
-
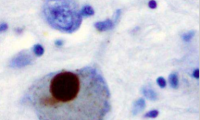
Identifying ‘hallmark’ Parkinson’s disease protein buildup could aid early detection, improved diagnosis and treatment
- Source: drugdu
- 513
- April 13, 2023
-
Newly merged manufacturers Meridian, Kindeva invest $100M in Missouri site
- Source: drugdu
- 336
- April 13, 2023
-
Moderna nabs a win in Arbutus patent case as appeals court upholds prior invalidation
- Source: drugdu
- 371
- April 13, 2023
-
Biden administration declares fentanyl laced with xylazine ‘an emerging threat’ in the US
- Source: drugdu
- 613
- April 13, 2023
-
EPA proposes new regulations on toxic gas used to sterilize spices and medical equipment
- Source: drugdu
- 421
- April 13, 2023
-
FDA, citing safety concerns, places partial hold on Merck KGaA’s MS drug
- Source: drugdu
- 567
- April 13, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.