-
FDA awards Biogen’s ALS drug Qalsody an accelerated approval, following its experts’ feedback this time
- Source: drugdu
- 457
- April 27, 2023
-
Novartis trims 10% of drug pipeline in research cutback
- Source: drugdu
- 660
- April 27, 2023
-
Biogen’s new strategy brings more pipeline cuts, but leaves deal options open
- Source: drugdu
- 443
- April 27, 2023
-
Implications for public health
- Source: drugdu
- 315
- April 27, 2023
-
High-protein diet counters adaptive thermogenesis in prediabetic individuals
- Source: drugdu
- 307
- April 27, 2023
-
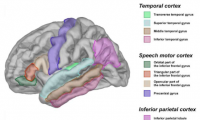
Language impairment in autism associated with gray matter volume
- Source: drugdu
- 316
- April 27, 2023
-
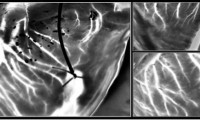
Laser speckle imaging can identify hearts suitable for transplantation
- Source: drugdu
- 477
- April 27, 2023
-
survey
- Source: drugdu
- 284
- April 27, 2023
-
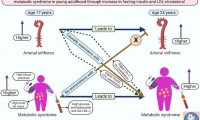
Arterial stiffness may be a novel risk factor for childhood and adolescent metabolic disease
- Source: drugdu
- 262
- April 27, 2023
-
Study shows no significant cognitive benefit of adhering to Mediterranean diets regardless of calorie intake
- Source: drugdu
- 280
- April 27, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.