-
Bayer to Sell its Brands to Denmark’s Leo Pharma
- Source: Reuters
- 859
- August 2, 2018
-
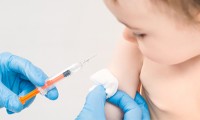
Chinese Vaccine Revoked, Parents Anxious
- Source: HealthThoroughfare
- 1,048
- August 2, 2018
-
Silencing Protein could Enhance Immunotherapy Efficacy in Cancer Tumors
- Source: MedicalXpress
- 1,092
- August 2, 2018
-
HiFiBiO Receives New Funding, Inks R&D Deal with Takeda
- Source: FierceBiotech
- 1,098
- August 2, 2018
-
$7.6M Granted to SkinVision to Expand Skin Cancer App
- Source: MobiHealthNews
- 1,055
- August 2, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.