-
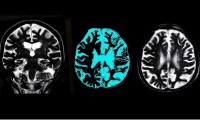
FDA Indicates Potential Full Approval for Eisai & Biogen’s Leqembi Ahead of Adcomm
- Source: drugdu
- 259
- June 10, 2023
-
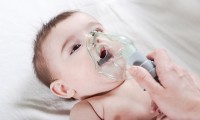
FDA advisers endorse antibody to protect against RSV in infants and some young toddlers
- Source: drugdu
- 378
- June 10, 2023
-
Neurotic people are more likely to suffer from mood swings, study finds
- Source: drugdu
- 243
- June 9, 2023
-
New study shows certain blood pressure drugs could boost the efficacy of cancer immunotherapy
- Source: drugdu
- 425
- June 9, 2023
-
Study
- Source: drugdu
- 295
- June 9, 2023
-
Philips faces new recall of Evo respirators, tagged Class I by FDA
- Source: drugdu
- 333
- June 9, 2023
-
FDA document outlines apparent agency support for full approval of Biogen, Eisai’s Leqembi
- Source: drugdu
- 384
- June 9, 2023
-
Johnson & Johnson, Legend file for expanded use of Carvykti after key trial win
- Source: drugdu
- 469
- June 9, 2023
-
Merck sues US government over Medicare drug price negotiation programme
- Source: drugdu
- 415
- June 9, 2023
-
GSK’s RSV vaccine approved by EC for older adults
- Source: drugdu
- 331
- June 9, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.