-
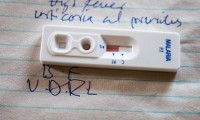
New POC Malaria Test 12 Times Faster Than Currently Available Laboratory-Based Tests
- Source: drugdu
- 101
- March 5, 2024
-
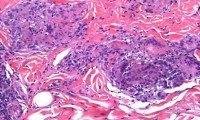
New Discoveries of Prostate Cancer Evolution Pave Way for Genetic Test
- Source: drugdu
- 168
- March 5, 2024
-
Profound Medical, Siemens Healthineers collab on ultrasound ablation
- Source: drugdu
- 91
- March 4, 2024
-
New Hantavirus Rapid Test Paves Way for Early Outbreak Control
- Source: drugdu
- 107
- March 2, 2024
-
New Alzheimer’s Detecting Blood Tests Perform across Broad Range of Races and Ethnicities
- Source: drugdu
- 124
- March 2, 2024
-
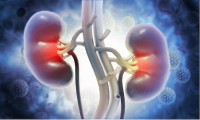
Simple Measurement Predicts Risk of Rapid Progression of Chronic Kidney Disease
- Source: drugdu
- 100
- February 29, 2024
-
Alzheimer’s Blood Test Could Soon Replace Invasive Spinal Taps and Brain Scans
- Source: drugdu
- 169
- February 28, 2024
-
Zdravookhraneniye 2024 Moscow Russia
- Source: drugdu
- 181
- February 27, 2024
-
February 23, 2024
- Source: drugdu
- 86
- February 27, 2024
-
Simple Blood Test Quickly and Accurately Diagnoses Potentially Life-Threatening Inflammatory Disease
- Source: drugdu
- 197
- February 26, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.