-
AstraZeneca’s Lynparza to dominate PARP inhibitors market with $4 bn in sales by 2027
- Source: https://www.expresspharma.in/astrazenecas-lynparza-to-dominate-parp-inhibitors-market-with-4-bn-in-sales-by-2027-globaldata/
- 167
- May 4, 2024
-
Six Biopharma Companies to Watch at MedCity INVEST Pitch Perfect Contest
- Source: drugdu
- 97
- May 2, 2024
-
Pfizer’s First Gene Therapy Approval Sets Up a Showdown With CSL in Hemophilia B
- Source: drugdu
- 75
- May 2, 2024
-
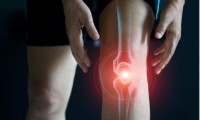
Blood Test Predicts Knee Osteoarthritis Eight Years Before Signs Appears On X-Rays
- Source: drugdu
- 116
- May 1, 2024
-
New CE-Marked Hepatitis Assays to Help Diagnose Infections Earlier
- Source: drugdu
- 121
- April 29, 2024
-
AI-Powered Digital Imaging System to Revolutionize Cancer Diagnosis
- Source: drugdu
- 112
- April 29, 2024
-
Pharma Asia 2024
- Source: drugdu
- 167
- April 29, 2024
-
Exo adds FDA-cleared AI tools to handheld ultrasound system
- Source: drugdu
- 111
- April 28, 2024
-
New Method Offers Sustainable Approach to Universal Metabolic Cancer Diagnosis
- Source: drugdu
- 176
- April 26, 2024
-
Blood Test Accurately Predicts Lung Cancer Risk and Reduces Need for Scans
- Source: drugdu
- 109
- April 26, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.