-
AWS, Hoppr Launch Model to Accelerate Generative AI Tools in Medical Imaging
- Source: drugdu
- 122
- November 28, 2023
-
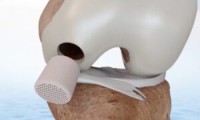
Smith+Nephew to buy CartiHeal and its knee cartilage healing tech
- Source: drugdu
- 138
- November 25, 2023
-
ChatGPT outperforms expert surgeon in answering rhinoplasty patient questions
- Source: drugdu
- 98
- November 22, 2023
-
FDA approves study of Versameb’s mRNA therapy in urinary incontinence
- Source: drugdu
- 173
- November 20, 2023
-
Spinal stimulation implant offers new hope for Parkinson’s disease treatment
- Source: drugdu
- 96
- November 13, 2023
-
Cannabis and derivatives show promise in reducing weight and waist circumference, with nuanced effects on body fat
- Source: drugdu
- 107
- November 10, 2023
-
Candel announces promising results for immunotherapy candidate in pancreatic cancer
- Source: drugdu
- 100
- November 8, 2023
-
CooperCompanies buys reproductive health products from Cook Medical after failed takeover
- Source: drugdu
- 162
- November 4, 2023
-
Merck’s Keytruda combination granted FDA approval for biliary tract cancer
- Source: drugdu
- 96
- November 4, 2023
-
FDA posts updated safety data on Bayer’s Essure, notes progress on improving study
- Source: drugdu
- 115
- November 2, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.