-
What Type of Medical Stretcher Should I Use?
- Source: Ddu
- 1,970
- August 13, 2018
-
One Boy’s Struggle with Epilepsy Leads to First Marijuana-Derived Medicine
- Source: NPR
- 976
- August 9, 2018
-
Alembic Pharma gets USFDA Approval for Ophthalmic Solution
- Source: MoneyControl
- 1,073
- August 8, 2018
-
Sun Pharma Launches Kapspargo Sprinkle to Treat Chest Pain & High Blood Pressure
- Source: MoneyControl
- 1,042
- August 7, 2018
-
Axiostat Becomes the First US FDA-Approved Wound Dressing Product from India
- Source: BusinessToday
- 1,112
- August 3, 2018
-
Adherium Receives FDA Clearance for Hailie Inhaler Sensor
- Source: MobiHealthNews
- 1,851
- August 1, 2018
-
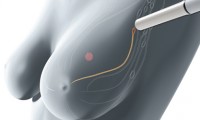
Endomag Breast Cancer Device Gains FDA Approval
- Source: The Verdict
- 1,135
- August 1, 2018
-
Elagolix soon to be Available for Endometriosis Treatment
- Source: NewScientist
- 1,083
- July 31, 2018
-
NuVasive gets FDA Approval for Pulse Spinal Surgical Automation Platform
- Source: MassDevice
- 435
- July 31, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.