-
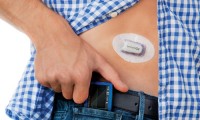
FDA clears new Dexcom CGM that requires no patient calibration earlier than expected
- Source: MedCityNews
- 1,158
- March 29, 2018
-
Bayer announces completion of rolling submission of New Drug Application in the U.S. for larotrectinib for the treatment of TRK fusion cancer
- Source: investor.bayer.de
- 844
- March 28, 2018
-
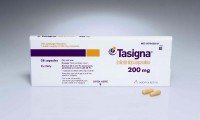
Novartis drug Tasigna® approved by FDA to treat children with rare form of leukemia
- Source: novartis
- 880
- March 26, 2018
-
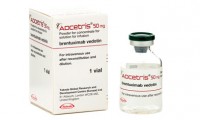
FDA expands approval of Adcetris for first-line treatment of Stage III or IV classical Hodgkin lymphoma in combination with chemotherapy
- Source: finance.yahoo
- 935
- March 22, 2018
-
FDA Approves Hizentra® (Immune Globulin Subcutaneous 20% Liquid) for the Treatment of Patients With Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Source: finance.yahoo
- 926
- March 20, 2018
-
Janssen Announces U.S. FDA Breakthrough Therapy Designation for Erdafitinib in the Treatment of Metastatic Urothelial Cancer
- Source: janssen
- 950
- March 19, 2018
-
FDA Grants Breakthrough Device Designation to Polyganics’ Liver and Pancreas Sealant Patch
- Source: polyganics
- 1,020
- March 15, 2018
-
Patient death forces FDA to slap a hold on study using combo from Advaxis, AstraZeneca — biotech’s shares plunge
- Source: endpts
- 798
- March 15, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.