Smiths Medical touts FDA nod, US launch of DeltaVan catheter
March 26, 2018
Source: MassDevice
 835
835
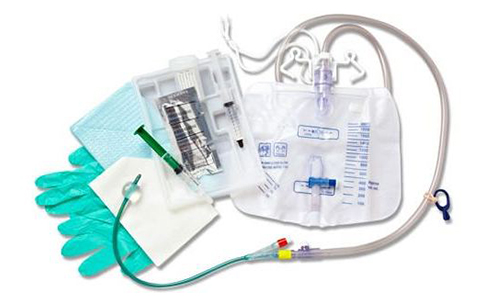
Smiths Medical today touted the FDA clearance and US launch of Delta Med’s DeltaVan closed system catheter.
The DeltaVen device is a next-gen peripheral IV catheter which offers the needle, extension tubing and options for a needleless connector. Smiths Medical said it will be available immediately in a variety of sizes and configurations.
Minneapolis, Minn.-based Smiths acts as the official distributor of Delta Med’s products.
“We are pleased to expand Smiths Medical’s portfolio with the DeltaVen closed system catheter and provide customers great options to successfully treat their patients,” prez & CEO Chris Holmes said in a prepared statement.
Earlier this month, Smiths Medical said it plans to distribute its CADD infusion systems to non-hospital-based providers through a preferred partnership with Medical Specialties Distributors.
By DduRead more on
- AZ’s Farxiga Gets FDA Priority Review For Heart Failure January 8, 2020
- Global Recall of CyPass Micro-Stent by Alcon September 3, 2018
- Join Drugdu.com’s Online Medical Devices Exhibition to Discover Global Trading Opportunities September 3, 2018
- 3 Ozone Generators for Improved Health August 30, 2018
- Basic Surgical Instrument – Extraction Forceps August 30, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



