-
Shares of biotech Juno, already up 50% on deal hopes, skyrocket on actual Celgene $9 billion offer
- Source: Cnbc
- 1,264
- January 24, 2018
-
Obsidian Therapeutics Announces $49.5 Million Series A Financing to Develop Controllable Cell and Gene Therapy Products
- Source: Businesswire
- 811
- December 8, 2017
-
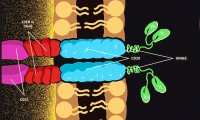
Stanford, NCI Researchers Discover New CAR-T Target With Early Study Data
- Source: biospace
- 913
- November 22, 2017
-
Novartis Slams Down $3.9B for This French Cancer Biotech
- Source: Biospace
- 1,024
- October 31, 2017
-
Kite’s Yescarta™ (Axicabtagene Ciloleucel) Becomes First CAR T Therapy Approved by the FDA
- Source: Businesswire
- 989
- October 20, 2017
-
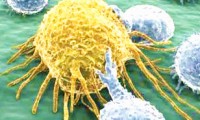
FDA Okays Another CAR T-Cell Therapy
- Source: medpagetoday
- 993
- October 19, 2017
-
Takeda and Noile-Immune Biotech Collaborate to Advance Next Generation CAR-T Cell Therapy Effective for Solid Tumors
- Source: Takeda
- 1,430
- September 6, 2017
-
Kite begins EU trial of CAR–T therapy
- Source: pharmatimes
- 1,300
- August 10, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.