-
Odds of Heart Attack Six Times Higher With Flu Diagnosis
- Source: drugdu
- 123
- April 19, 2023
-
Pre- and post-surgical immunotherapy-based treatment significantly improved lung cancer outcomes
- Source: drugdu
- 124
- April 18, 2023
-
Adding new vaccine type to leading immunotherapy dramatically reduced melanoma recurrence
- Source: drugdu
- 230
- April 17, 2023
-
FDA, citing safety concerns, places partial hold on Merck KGaA’s MS drug
- Source: drugdu
- 200
- April 13, 2023
-
The virological characteristics of XBB.1.16
- Source: drugdu
- 120
- April 13, 2023
-
‘This Is a First’
- Source: drugdu
- 150
- April 12, 2023
-
Moderna cancer vaccine takes positive step
- Source: drugdu
- 112
- April 12, 2023
-
Pfizer and Moderna’s COVID-19 vaccines offer lasting protection
- Source: drugdu
- 175
- April 12, 2023
-
New study shows SARS-CoV-2 infection accelerates the progression of dementia
- Source: drugdu
- 164
- April 7, 2023
-
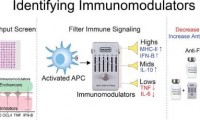
Immunomodulators boost vaccine response and reduce inflammation, finds study
- Source: drugdu
- 150
- April 6, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.