-
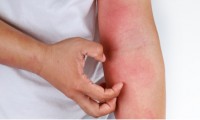
Sanofi’s amlitelimab shows promise in phase 2b atopic dermatitis trial
- Source: drugdu
- 190
- June 30, 2023
-
Sanofi’s $1.4B Bet Pays Off with Promising Phase II Eczema Data
- Source: drugdu
- 159
- June 29, 2023
-
Pfizer’s Litfulo approved by FDA as first treatment for adolescents with severe alopecia areata
- Source: drugdu
- 120
- June 29, 2023
-
Global study shows loneliness can shorten life spans
- Source: drugdu
- 105
- June 28, 2023
-
Amgen’s supplemental Biologics License Application for Blincyto approved by FDA
- Source: drugdu
- 108
- June 27, 2023
-
Empress Therapeutics emerges with $50m to fund small molecule development
- Source: drugdu
- 122
- June 23, 2023
-
Tessa Therapeutics Hodgkin’s Lymphoma CAR-T therapy shows promising Phase I data
- Source: drugdu
- 170
- June 20, 2023
-
Race for universal flu vaccine ramps up as Osivax kicks off Phase IIa trial
- Source: drugdu
- 103
- June 20, 2023
-
Scientists develop universal donor stem cell therapy to treat degenerative brain diseases in a preclinical study
- Source: drugdu
- 139
- June 17, 2023
-
Valneva Reports Strong Phase III Chikungunya Vaccine Data After Merck’s Exit from Race
- Source: drugdu
- 117
- June 15, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.