-
GSK outlines deal to send cell therapies back to Adaptimmune
- Source: drugdu
- 262
- April 12, 2023
-
Pfizer and Moderna’s COVID-19 vaccines offer lasting protection
- Source: drugdu
- 175
- April 12, 2023
-
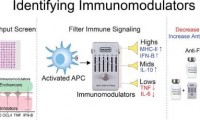
Immunomodulators boost vaccine response and reduce inflammation, finds study
- Source: drugdu
- 150
- April 6, 2023
-
SARS-CoV-2 infection weakens immune-cell response to vaccination
- Source: drugdu
- 129
- April 6, 2023
-
mRNA vaccine beats infection for key defense against COVID-19, Stanford Medicine scientists find
- Source: drugdu
- 144
- March 31, 2023
-
Roche Holding AG Receives FDA Approval for New Cancer Treatment
- Source: drugdu
- 147
- March 24, 2023
-
Frozen Strawberries Recalled Due to Hepatitis A Outbreak
- Source: drugdu
- 105
- March 23, 2023
-
FDA Grants Breakthrough Therapy Designation to Pfizer’s Investigational Alzheimer’s Disease Treatment
- Source: drugdu
- 252
- March 22, 2023
-
Big news! Hengrui ADC innovative drug SHR-A1811 new indication to be included in breakthrough therapy species
- Source: drugdu
- 143
- March 20, 2023
-
NICE questions value and efficacy of five Covid-19 drugs with rejection
- Source: drugdu
- 152
- March 17, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.