-
Look out, GSK. Pfizer has its adult RSV vaccine approval and is raring to launch
- Source: drugdu
- 125
- June 5, 2023
-
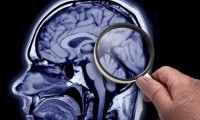
Medicare details plan to cover Alzheimer’s treatments
- Source: drugdu
- 115
- June 4, 2023
-
CPhI Worldwide Europe
- Source: drugdu
- 1,054
- June 1, 2023
-
Cats can play a role in transmitting COVID-19, finds new study
- Source: drugdu
- 138
- June 1, 2023
-
Study maps immune responses produced by COVID-19 vaccination in First Nations population
- Source: drugdu
- 118
- May 31, 2023
-
AbbVie and Genmab’s blood cancer therapy granted FDA accelerated approval
- Source: drugdu
- 117
- May 24, 2023
-
Phase I trial demonstrates first pharmacological treatment able to improve cardiac function in stiff-heart syndrome
- Source: drugdu
- 112
- May 22, 2023
-
CDC
- Source: drugdu
- 115
- May 22, 2023
-
SCOTUS hands win to Sanofi, Regeneron in long-running PCSK9 feud with Amgen
- Source: drugdu
- 120
- May 21, 2023
-
Pfizer to raise $31 billion in debt offering to fund Seagen acquisition, SEC filing shows
- Source: drugdu
- 129
- May 19, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.