-
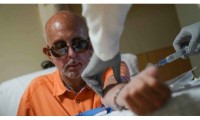
Takeda Withdraws Dengue Vaccine Application After FDA Request for More Data
- Source: drugdu
- 124
- July 14, 2023
-
BeiGene Signs $1.3bn Deal with DualityBio for Investigational Solid Tumour ADC
- Source: drugdu
- 127
- July 14, 2023
-
FDA Puts Clinical Hold on Hemogenyx’s CAR-T AML Therapy
- Source: drugdu
- 144
- July 12, 2023
-
CMS Expands Medicare Coverage for Leqembi After FDA Traditional Approval
- Source: drugdu
- 131
- July 11, 2023
-
Sales of Alzheimer’s Drug Leqembi may be Slow Initially But Could Pick up in 2024
- Source: drugdu
- 117
- July 11, 2023
-
Astellas Gains FDA Priority Review for Gastric Cancer Therapy BLA
- Source: drugdu
- 121
- July 11, 2023
-
Takeda and F-star announce immunotherapy partnership worth over $1bn
- Source: drugdu
- 110
- July 10, 2023
-
FDA grants traditional approval for Eisai-Biogen’s Alzheimer’s therapy
- Source: drugdu
- 109
- July 9, 2023
-
Boehringer’s spesolimab shows promise in generalised pustular psoriasis prevention
- Source: drugdu
- 199
- July 6, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.