-
FDA Warns of Unapproved Vaginal ‘Rejuvenation’ Procedures
- Source: Medpagetoday
- 845
- August 1, 2018
-
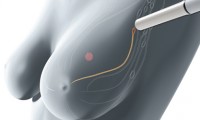
Endomag Breast Cancer Device Gains FDA Approval
- Source: The Verdict
- 780
- August 1, 2018
-
NuVasive gets FDA Approval for Pulse Spinal Surgical Automation Platform
- Source: MassDevice
- 188
- July 31, 2018
-
Contamination of Synthetic Cannabis with Rat Poison Affects Blood Supply
- Source: NBC News
- 844
- July 30, 2018
-
FDA Approves Healthy.io Smartphone Camera-based Home Urine Analysis
- Source: MobiHealthNews
- 586
- July 27, 2018
-
Siemens gets FDA Clearance for Troponin Assay
- Source: FierceBiotech
- 1,021
- July 27, 2018
-
Valsartan Based Drugs Recalled by FDA
- Source: HealthLine
- 865
- July 26, 2018
-
Mersana’s Lead Cancer ADC Held Up by FDA for Patient’s Death
- Source: FierceBiotech
- 196
- July 25, 2018
-
Karyopharm Seeks FDA Approval for Selinexor Drug for Multiple Myeloma
- Source: MedCityNews
- 410
- July 24, 2018
-
Siga Technologies Wins FDA Approval for Tecovirimat to Treat Smallpox
- Source: DrugDeliveryBusiness
- 725
- July 17, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.