-
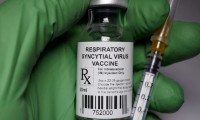
FDA advisors recommend Pfizer’s RSV vaccine for infants but raise safety concerns
- Source: drugdu
- 115
- May 20, 2023
-
Intercept’s latest NASH bid in jeopardy after FDA questions drug’s efficacy, safety
- Source: drugdu
- 145
- May 19, 2023
-
Allergan’s new skin-smoothing product gets FDA nod—with novel delivery mechanism
- Source: drugdu
- 120
- May 19, 2023
-
`FDA advisory committee backs Sarepta’s Duchenne muscular dystrophy gene therapy
- Source: drugdu
- 111
- May 18, 2023
-
FDA Approves First-Of-Its Kind Drug to Ease Menopause Symptoms
- Source: drugdu
- 150
- May 16, 2023
-
FDA greenlights a new type of drug for menopausal hot flashes
- Source: drugdu
- 120
- May 16, 2023
-
After costly setback, Astellas’ menopause drug crosses FDA finish line
- Source: drugdu
- 111
- May 16, 2023
-
Chiesi, Protalix’s PRX-102 secure FDA nod to treat Fabry disease
- Source: drugdu
- 142
- May 15, 2023
-
ImmunityBio gets manufacturing-related FDA snub on cancer drug, shares crash
- Source: https://seekingalpha.com/news/3969942-immunitybio-stock-crashes-after-fda-snub-cancer-therapy
- 121
- May 14, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.