-
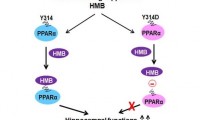
Bodybuilding supplement may help stave off progression of Alzheimer’s
- Source: drugdu
- 348
- July 22, 2023
-
Sanofi Puts Up $125M to Partner on Oral Drug That Could Be Dupixent’s Successor
- Source: drugdu
- 499
- July 22, 2023
-
Shifting tides for weight loss drugs
- Source: drugdu
- 259
- July 22, 2023
-
How doctors are learning to work with GPT-4
- Source: drugdu
- 309
- July 22, 2023
-
J&J Flags Potential Interference Between Heart Pump and TAVR Stents in Recall
- Source: drugdu
- 427
- July 21, 2023
-
Study Shows How NSAIDs Exacerbate C. Difficile Infections
- Source: drugdu
- 334
- July 21, 2023
-
Phase III Data Show Keytruda’s Promise in Earlier-Stage Cervical Cancer
- Source: drugdu
- 401
- July 21, 2023
-
J&J, Astellas Join Pharma Lawsuits Over Medicare Drug Price Negotiations
- Source: drugdu
- 270
- July 21, 2023
-
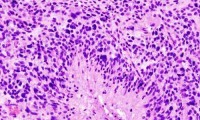
Researchers Report on Inhibitory Effect of Glioblastoma Signals to Macrophages
- Source: drugdu
- 266
- July 21, 2023
-
Medicaid MCOs Denied 1 out of 8 Prior Authorization Requests in 2019
- Source: drugdu
- 352
- July 21, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.