-
Mirati’s Flagship KRAS Inhibitor Krazati Gets Rebuffed in Europe
- Source: drugdu
- 366
- July 25, 2023
-
ADC Therapeutics Pulls the Plug on Zynlonta Study After Partial FDA Hold
- Source: drugdu
- 380
- July 25, 2023
-
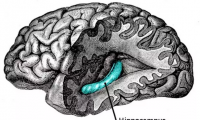
Study Explores the Emergence of Predictive Models in the Hippocampus
- Source: drugdu
- 279
- July 25, 2023
-
VR Can Alleviate Brain Tumor Patients’ Anxiety During Treatment, Study Finds
- Source: drugdu
- 258
- July 25, 2023
-
Muscle-Building Supplement May Help Protect Memory and Prevent Alzheimer’s Disease
- Source: drugdu
- 425
- July 24, 2023
-
Bariatric Surgery Slowdown Nips Intuitive Surgical’s Q2 Procedure Growth
- Source: drugdu
- 362
- July 24, 2023
-
Pfizer and Flagship Pioneering Sign Drug Discovery Partnership Worth up to $7bn
- Source: drugdu
- 645
- July 24, 2023
-
Kenvue Shares Fall Even as J&J Spinoff Beats Estimates in First Quarterly Earnings Since IPO
- Source: drugdu
- 336
- July 24, 2023
-
J&J’s Darzalex Stays on its Upward Trajectory as Company Adds $1B to Annual Sales Projection
- Source: drugdu
- 358
- July 24, 2023
-
Sandoz Invests $90m to Build a Biosimilar Centre in Slovenia
- Source: drugdu
- 321
- July 24, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.