-
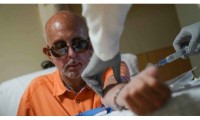
Sales of Alzheimer’s Drug Leqembi may be Slow Initially But Could Pick up in 2024
- Source: drugdu
- 118
- July 11, 2023
-
New program offers hiking adventures for people with younger-onset Alzheimer’s
- Source: drugdu
- 117
- July 10, 2023
-
FDA grants traditional approval for Eisai-Biogen’s Alzheimer’s therapy
- Source: drugdu
- 110
- July 9, 2023
-
With full Alzheimer’s approval in hand, Eisai and Biogen kick off Leqembi’s launch in earnest
- Source: drugdu
- 112
- July 8, 2023
-
Fluctuating blood lipid levels linked with higher risk of Alzheimer’s disease, study finds
- Source: drugdu
- 117
- July 7, 2023
-
Missed trial protocols, fabricated emails and failed endpoint mar BioXCel’s Alzheimer’s agitation readout
- Source: drugdu
- 106
- July 2, 2023
-
BioXcel’s Positive Alzheimer’s Agitation Data Overshadowed by Principal Investigator Fabrications
- Source: drugdu
- 108
- July 1, 2023
-
Eisai, Biogen’s Alzheimer’s disease drug Leqembi passes muster at FDA adcomm
- Source: drugdu
- 113
- June 13, 2023
-
Axol and StrataStem link up for Alzheimer’s disease trial
- Source: drugdu
- 127
- June 10, 2023
-
Lawmakers worry Medicare Alzheimer’s plan won’t ensure access to new treatments
- Source: drugdu
- 168
- June 8, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.