-
Schizophrenia Trial Failure Ends Acadia’s Efforts to Expand Use of Its Flagship Drug
- Source: drugdu
- 155
- March 16, 2024
-
Roche partners with Cardiff researchers to uncover new research into dementia
- Source: drugdu
- 120
- March 16, 2024
-
MIT study reveals non-invasive treatment holds promise for treating ‘chemo brain’
- Source: drugdu
- 134
- March 12, 2024
-
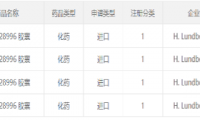
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
- Source: drugdu
- 159
- March 7, 2024
-
Researchers develop brain-age prediction tool for diagnosis of neurological diseases
- Source: drugdu
- 90
- March 7, 2024
-
Researchers develop terahertz biosensor to detect skin cancer for early diagnosis
- Source: drugdu
- 157
- February 28, 2024
-
Sanofi, Denali Neuro Drug Fails Mid-Stage Trial in ALS; MS Study Is Continuing
- Source: drugdu
- 152
- February 26, 2024
-
Ultra-Sensitive Blood Test Reflects Brain Damage and Predicts Functional Outcomes after Ischemic Stroke
- Source: drugdu
- 166
- February 23, 2024
-
Otsuka wins FDA breakthrough therapy designation for IgAN drug
- Source: drugdu
- 92
- February 19, 2024
-
New programme shown to help dementia patients live independently
- Source: https://pharmatimes.com/news/new-programme-shown-to-help-dementia-patients-live-independently/
- 92
- February 11, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.