-
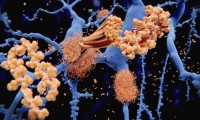
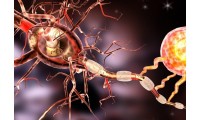
Researchers discover how neurons die in Alzheimer’s disease
- Source: drugdu
- 108
- September 22, 2023
-
Anavex’s Blarcamesine Slows Cognitive Decline in Alzheimer’s Patients
- Source: drugdu
- 107
- September 19, 2023
-
New Biomarker Tests Aid Quick, Accurate Diagnosis of Alzheimer’s Disease
- Source: drugdu
- 188
- September 6, 2023
-
Quest Launches Alzheimer’s Beta-Amyloid Blood Test for Consumers
- Source: drugdu
- 216
- August 3, 2023
-
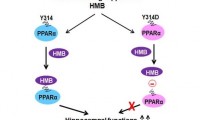
Muscle-Building Supplement May Help Protect Memory and Prevent Alzheimer’s Disease
- Source: drugdu
- 206
- July 24, 2023
-
Bodybuilding supplement may help stave off progression of Alzheimer’s
- Source: drugdu
- 116
- July 22, 2023
-
Alnylam Presents Promising Results for RNAi Therapeutic in Alzheimer’s Disease at AAIC
- Source: drugdu
- 120
- July 20, 2023
-
Acumen Shares Positive Results for Investigational Alzheimer’s Disease Therapy at AAIC
- Source: drugdu
- 124
- July 19, 2023
-
Eli Lilly Strengthens the Case for Going Early in Alzheimer’s Treatment
- Source: drugdu
- 114
- July 19, 2023
-
Eisai, Biogen’s Leqembi May Face Rollout Hurdles Now, but Experts Still Like the Alzheimer’s Drug
- Source: drugdu
- 102
- July 15, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.