-
Simple Blood Test Could Detect Risk of Viral Infection-Induced Cardiac Arrest
- Source: drugdu
- 87
- March 9, 2024
-
FDA Approves Opdivo Plus Chemotherapy for Frontline Treatment of Unresectable, Metastatic Urothelial Carcinoma
- Source: drugdu
- 124
- March 9, 2024
-
Gilead and Merus enter trispecific antibody discovery deal
- Source: drugdu
- 104
- March 9, 2024
-
【EXPERT Q&A】How is the quality inspection and quarantine carried out in the import and export industry?
- Source: drugdu
- 179
- March 8, 2024
-
Automated Multiplexing Molecular Diagnostic Platform Revolutionizes Infectious Disease Diagnostics
- Source: drugdu
- 135
- March 8, 2024
-
Researchers use collected stem cells to grow cell models to advance prenatal medicine
- Source: drugdu
- 104
- March 8, 2024
-
Simple Blood Test Measures Repetitive DNA for Early Cancer Detection
- Source: drugdu
- 141
- March 7, 2024
-
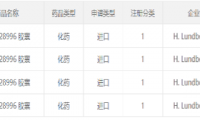
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
- Source: drugdu
- 154
- March 7, 2024
-
Shuangcheng Pharmaceuticals Octreotide Acetate Injection Obtained Certificate of Drug Registration
- Source: drugdu
- 119
- March 7, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.