-
KCL project receives £100,000 UK government funding to boost AI-assisted healthcare
- Source: drugdu
- 78
- April 9, 2024
-
Johnson & Johnson Set to Acquire Shockwave Medical, Aiming to Strengthen Company’s MedTech Division
- Source: drugdu
- 81
- April 8, 2024
-
Beckman Coulter receives FDA warning letter
- Source: drugdu
- 83
- April 8, 2024
-
Legend Biotech’s “CARVYKTI®” was approved for second-line indications in the United States
- Source: drugdu
- 106
- April 8, 2024
-
Key mechanism controlling bone marrow stem cells could lead to new therapies
- Source: drugdu
- 101
- April 8, 2024
-
Bessel launches accelerator program for medtech startups
- Source: drugdu
- 132
- April 7, 2024
-
CellVoyant secures £7.6 million AI stem cell boost
- Source: drugdu
- 102
- April 7, 2024
-
Aston University receives £10m for membrane institute
- Source: drugdu
- 132
- April 7, 2024
-
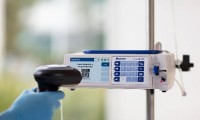
Baxter receives FDA clearance for delayed Novum IQ infusion pump
- Source: drugdu
- 135
- April 5, 2024
-
Alebund receives ODD for investigational drug AP303
- Source: drugdu
- 123
- April 5, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.