Baxter receives FDA clearance for delayed Novum IQ infusion pump
April 5, 2024
Source: drugdu
 418
418
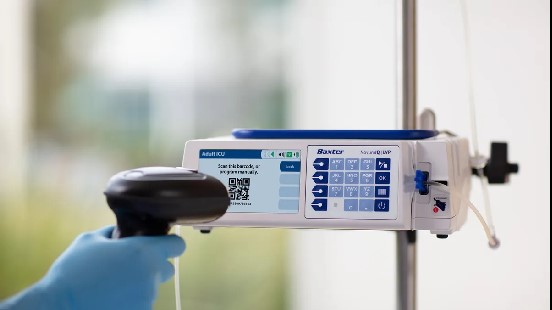
Dive Brief
Baxter has received 510(k) clearance for its large volume infusion pump (LVP) Novum IQ, the company said Monday.
The clearance follows three years of talks with the Food and Drug Administration, in which time a delay to the authorization drove Baxter to wipe $100 million in sales off its forecast in 2022. A Baxter spokesperson said in an email that the company will update 2024 guidance on its upcoming first-quarter earnings call and expects Novum IQ to be a meaningful contributor to future growth.
Evercore ISI analysts said the success of Baxter’s Spectrum pumps and the return of BD’s Alaris to the market means the $100 million annual sales assumption no longer applies. Even so, Evercore and Stifel analysts called the clearance positive for Baxter in notes to investors.
Dive Insight
The FDA identified an issue with Baxter’s filing for clearance of the LVP version of Novum IQ in 2022 and put the 510(k) on hold. At the time, Evercore analysts said the device could receive clearance in the first quarter of 2023. Ultimately, Baxter made its final resubmission to the FDA in the first quarter of 2023, and then waited another year to receive clearance.
The LVP market changed while Novum IQ was under FDA review. Supply constraints meant Baxter was initially unable to ramp up production of its Spectrum pump to fill the void created by the delay to Novum IQ. Baxter has since cleared those constraints and established Spectrum as a growing product.
Clare Trachtman, vice president of investor relations, said at an investor event in March that Baxter was a “net share gainer” last year, adding that she expects Spectrum to gain share again in 2024.
Evercore analysts estimate that Baxter’s guidance assumes 20% growth in Spectrum sales. The Evercore analysts concluded that because of Spectrum’s success, the $100 million estimate of Novum IQ’s impact no longer applies and the new pump is “perhaps a small incremental contributor” to Baxter’s performance. Meanwhile, Trachtman said Novum IQ has advantages that provide a greater opportunity to gain share with the new device.
“Spectrum is a great pump, it’s got some of the most advanced safety features out there, it’s a workforce pump. So, we’ve done really well at protecting our installed base, while gaining some share with this. We believe the opportunity set does open up a bit more with Novum,” Trachtman said.
The delay to Novum IQ also enabled BD to return Alaris to the market before Baxter launched its rival pump. Evercore analysts said the Alaris authorization “minimizes [Baxter’s] scope for share gains as the major competitor is back on market.”
Stifel analysts were more upbeat about the clearance. “Management has repeatedly-emphasized that the Novum LVP could drive meaningful annual sales contributions, ranging from $25 million to $100 million, which currently are not reflected in 2024 guidance and Street numbers,” the analysts wrote. “Thus, we believe that following Baxter’s 1Q24 earnings report, 2024 consensus numbers potentially could be moving higher.”
Stifel analysts assume the “commercial rollout will begin gradually, and ramp more meaningfully as the year progresses.” The analysts left their estimates of Baxter’s financial results unchanged while waiting for the company to share more details of the commercial timeline and sales expectations. Baxter said the pump is “available to order in the U.S.” after the Stifel analysts published their note.
Source:
https://www.medtechdive.com/news/baxter-novum-iq-infusion-pump-fda-clearance/711948/
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



