-
Mabwell reaches the Oversea Market of South Asia and Africa
- Source: drugdu
- 180
- March 29, 2024
-
a global network for coronaviruses
- Source: drugdu
- 105
- March 29, 2024
-
LifeArc provides £750,000 fund for clinical trial to treat neuroferritinopathy
- Source: drugdu
- 117
- March 29, 2024
-
PhaSER Biomedical and the Sanders TDI partner for clinical drug discovery research
- Source: drugdu
- 92
- March 29, 2024
-
Merck Drug for Heart and Lung Disorder Wins a First-in-Class FDA Approval
- Source: drugdu
- 89
- March 28, 2024
-
Viking Therapeutics Reveals Positive Results from Oral Obesity Drug
- Source: drugdu
- 121
- March 28, 2024
-
New Genomic Method Helps Diagnose Patients with Unexplained Kidney Disease
- Source: drugdu
- 81
- March 28, 2024
-
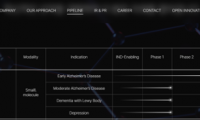
$770 Million! AriBio authorizes Marketing Rights for a Drug to Treat Alzheimer’s Disease in China
- Source: drugdu
- 135
- March 28, 2024
-
Inizio Engage and Applied Driving partner to support clinical workers with safety app
- Source: drugdu
- 85
- March 28, 2024
-
Researchers identify group of biological markers found in high levels in TB patients
- Source: drugdu
- 132
- March 28, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.