-
Capital Rx, Prime Therapeutics Form Strategic Alliance
- Source: drugdu
- 120
- March 25, 2024
-
Groundbreaking pTau217 Blood Test as Accurate as Brain Imaging or CSF Testing in Diagnosing Alzheimer’s
- Source: drugdu
- 150
- March 23, 2024
-
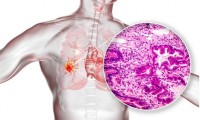
AI Predicts Cancer Spreading To Brain from Lung Biopsy Images
- Source: drugdu
- 101
- March 23, 2024
-
FDA Approves Johnson & Johnson’s Edurant PED for Pediatric Patients With HIV
- Source: drugdu
- 144
- March 23, 2024
-
CPhI India 2024
- Source: drugdu
- 151
- March 22, 2024
-
【EXPERT Q&A】What is FDA Food grade certification?
- Source: drugdu
- 114
- March 22, 2024
-
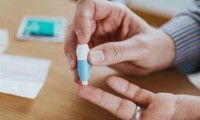
POC Finger-Prick Blood Test Determines Risk of Neutropenic Sepsis in Patients Undergoing Chemotherapy
- Source: drugdu
- 92
- March 22, 2024
-
FDA Approves Idorsia’s Tryvio for Once-Daily Treatment of Difficult-to-Control Hypertension
- Source: drugdu
- 97
- March 22, 2024
-
Startup Lands $150M for Delicate Dance Between Cancer Cells & Immune Cells
- Source: drugdu
- 86
- March 22, 2024
-
Henlius’ Trastuzumab Approved in Brazil, a Semi-Exclusive License Area, in Latin America
- Source: drugdu
- 93
- March 22, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.