$770 Million! AriBio authorizes Marketing Rights for a Drug to Treat Alzheimer’s Disease in China
March 28, 2024
Source: drugdu
 342
342
 It’s reported according to businesswire, AriBio recently announced that it has signed a licensing agreement for the exclusive marketing rights of AR1001, an investigational drug for the treatment of early Alzheimer's disease, in China at a price of up to US$770 million. Considering the market competition and sales strategy of Alzheimer's disease drugs in China, the licensee requested that no disclosure be made until the agreed time.
It’s reported according to businesswire, AriBio recently announced that it has signed a licensing agreement for the exclusive marketing rights of AR1001, an investigational drug for the treatment of early Alzheimer's disease, in China at a price of up to US$770 million. Considering the market competition and sales strategy of Alzheimer's disease drugs in China, the licensee requested that no disclosure be made until the agreed time.
The agreement includes an upfront non-refundable payment of 120 billion won (approximately US$90 million), with a total transaction value of up to 5.59 billion yuan (approximately US$770 million), including milestone payments and additional royalties. Upfront payments will begin in mid-2024.
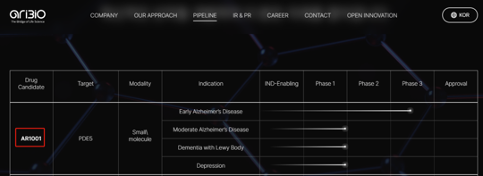
AR1001 is a phosphodiesterase type 5 (PDE5) inhibitor in development and an investigational oral drug for the treatment of Alzheimer's disease. Pre-clinical studies have confirmed that AR1001 can inhibit neuronal apoptosis and restore synaptic plasticity, thus having neuroprotective effects. AR1001 also demonstrated a significant reduction in hyperphosphorylated tau protein in preclinical models and phase II clinical trial.
AR1001-ADP3-US01 (NCT05531526) is a Phase III double-blind, randomized, placebo-controlled, multicenter trial designed to evaluate the efficacy and safety of AR1001 over 52 weeks in patients with early-stage Alzheimer's disease. The study launched in the United States in December 2022 and expanded to other countries in 2023. In addition, the clinical trial is awaiting regulatory approval in China and the European Union.
In view of the huge market demand for Alzheimer's disease, it is regarded as the "crown jewel" by the drug research and development community. Currently, approximately 50 million people worldwide are suffering from Alzheimer's disease, and this number is expected to increase to 152 million by 2050. Currently, the global annual cost of treating and caring for Alzheimer's disease patients has reached US$1 trillion, and this figure will double the current amount by 2030. This shows that Alzheimer's disease drugs will have a broad market. .
Statistics shows that there are 9.83 million AD patients and 38.77 million mild cognitive impairment (MCI) patients in China among people over the age of 60, and reports predict that by 2025, there will be 15.5 million Alzheimer's disease patients, and by 2030 it will further increase to 19.5 million people.
As the number of Alzheimer's patients in China continues to increase, many well-known pharmaceutical companies are seeking to develop safe and effective oral treatments for Alzheimer's disease. To date, AriBio has partnered with Samjin Pharmaceutical, granting it 100 billion won of exclusive sales rights in South Korea. Now, this cooperation has been extended to China.
Matthew (JaiJun) Choung, CEO and Chairman of AriBio, said: "This transaction demonstrates our unwavering commitment to developing effective treatments for Alzheimer's disease, and our partners will help make AR1001 available in key regions in Asia successfully. We will continue to discuss with other potential partners in Asian countries, the Middle East, South America, as well as Europe and the United States to accumulate advantages for our AR1001 project."
According to data, China's Alzheimer's disease diagnosis market size in 2020 was approximately 21.75 billion yuan, and it is expected that this market will reach 26.02 billion yuan by 2025.
There are few pharmaceutical companies in the domestic AD track, which belongs to the blue ocean track with relatively high demand and low competition. On January 9 this year, according to the official website of the State Food and Drug Administration: Japan's Eisai's Lencanezumab injection was approved for marketing for the treatment of mild cognitive impairment and mild dementia caused by Alzheimer's disease.
Domestic companies such as Simcere Pharmaceuticals, Hengrui Pharmaceuticals, and Hisun Pharmaceuticals are actively conducting research and development in this field, trying to get a share of the pie. At present, Qilu Pharmaceutical's memantine hydrochloride orally dissolving film has been approved by the NMPA for marketing, Hisun Pharmaceutical's AD-35 is in the second phase clinical stage, Simcere Pharmaceutical's PQ912 is in the second phase clinical stage, and Hengrui Pharmaceutical's SHR-1707 is in the first phase. clinical stage.
https://news.yaozh.com/archive/42378.html
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



