-
Senseonics gets FDA clearance to pair CGM implant with insulin pumps
- Source: https://www.medtechdive.com/news/senseonics-fda-clearance-integrated-cgm/714804/
- 81
- May 3, 2024
-
WuXi Biologics Releases 2023 ESG Report Demonstrating Strong Sustainability Commitment
- Source: drugdu
- 88
- May 2, 2024
-
ETH Zurich researchers use AI to develop drug molecules based on protein structures
- Source: drugdu
- 83
- May 1, 2024
-
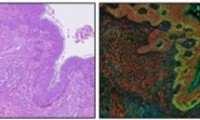
AI-Powered Digital Imaging System to Revolutionize Cancer Diagnosis
- Source: drugdu
- 112
- April 29, 2024
-
Chiatai Tianqing’s Class 1 Innovative Drug TQA3038 (siRNA) Completes Phase I Clinical Study
- Source: drugdu
- 140
- April 29, 2024
-
Exo adds FDA-cleared AI tools to handheld ultrasound system
- Source: drugdu
- 111
- April 28, 2024
-
Sanders takes aim at US drug prices of Novo’s Ozempic and Wegovy
- Source: drugdu
- 76
- April 28, 2024
-
New Method Offers Sustainable Approach to Universal Metabolic Cancer Diagnosis
- Source: drugdu
- 176
- April 26, 2024
-
FDA Drug Approval Marks a New Day for Treating Pediatric Brain Cancer
- Source: drugdu
- 80
- April 26, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.