-
NHS urges more young people to take up HPV vaccine
- Source: drugdu
- 286
- January 30, 2024
-
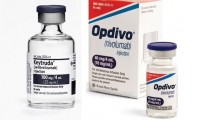
Merck’s Keytruda extends life for kidney cancer patients after surgery, while Bristol’s Opdivo fails again
- Source: drugdu
- 285
- January 30, 2024
-
J&J, Merck CEOs dodge subpoenas by agreeing to testify at Senate committee’s pricing hearing
- Source: drugdu
- 342
- January 30, 2024
-
Drug shortages challenge type 2 diabetes patients in the UK
- Source: drugdu
- 366
- January 30, 2024
-
New Tests to Help Predict Disease Progression in Ulcerative Colitis
- Source: drugdu
- 386
- January 27, 2024
-
First of Its Kind Software Uses AI and ML for Personalized Disease Prediction
- Source: drugdu
- 373
- January 27, 2024
-
Bladder Cancer Therapies Developer Raises $380M in the First Biotech IPO of 2024
- Source: drugdu
- 318
- January 27, 2024
-
Key Trends from the 2024 J.P. Morgan Healthcare Conference
- Source: drugdu
- 382
- January 27, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.