-
Biontech and Genevant Sciences Join Hands to Roll Out Rare Disease Drugs
- Source: Pharmaceutical Technology
- 1,020
- July 16, 2018
-
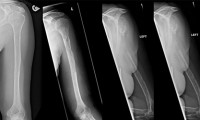
Woman’s Bone Vanished into Thin Air!
- Source: LiveScience
- 1,012
- July 12, 2018
-
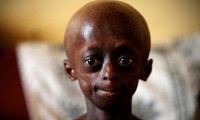
Therapy Against Progeria
- Source: Ddu
- 832
- June 7, 2018
-
Having Back Pain? Know If It Is Cauda Equina Syndrome
- Source: drugdu
- 787
- May 28, 2018
-
Shire Announces Sale of Oncology Business to Servier for $2.4 Billion
- Source: finance.yahoo
- 838
- April 17, 2018
-
Dova Pharmaceuticals Signs Exclusive Distribution Agreement with Fosun Pharma For Mainland China and Hong Kong
- Source: finance.yahoo
- 969
- March 21, 2018
-
Enzychem Lifesciences Announces the FDA Orphan Drug Designation Granted to EC-18 on Acute Radiation Syndrome (ARS)
- Source: china-pharmacy
- 1,066
- February 1, 2018
-
Sanofi to acquire Bioverativ for $11.6 billion
- Source: Marketwatch
- 735
- January 24, 2018
-
FDA awards 15 grants for clinical trials to stimulate product development for rare diseases
- Source: fda.gov
- 865
- October 9, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.