-
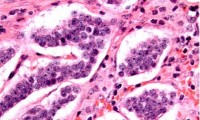
New Protein Biomarker to Help Develop Blood-Based Tests for Aggressive Neuroendocrine Carcinomas
- Source: drugdu
- 112
- February 19, 2024
-
Changing protocol language and site actions are key to making trials inclusive
- Source: drugdu
- 93
- February 16, 2024
-
NHS launches BRCA testing programme for people of Jewish descent
- Source: drugdu
- 129
- February 16, 2024
-
Simple Blood Test Can Predict Heart Attack Risk within Six Months
- Source: https://www.labmedica.com/molecular-diagnostics/articles/294800241/simple-blood-test-can-predict-heart-attack-risk-within-six-months.html
- 155
- February 15, 2024
-
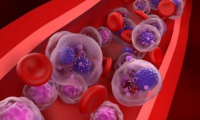
Hemogenyx gets reprieve as FDA lifts clinical hold on AML CAR-T therapy
- Source: https://www.pharmaceutical-technology.com/news/hemogenyx-gets-reprieve-as-fda-lifts-clinical-hold-on-aml-car-t-therapy/?cf-view
- 150
- February 15, 2024
-
Alys Pharmaceuticals announces launch with $100m funding
- Source: https://www.pharmaceutical-technology.com/news/alys-pharmaceuticals-announces-launch/?cf-view
- 74
- February 13, 2024
-
Study reveals AI can predict patients’ survival in glioblastoma
- Source: https://pharmat.com/news/study-reveals-ai-can-predict-patients-survival-in-glioblastoma/
- 95
- February 12, 2024
-
Study reveals SARS-CoV-2 can infect dopamine neurons causing senescence
- Source: https://news.weill.cornell.edu/news/2024/01/sars-cov-2-can-infect-dopamine-neurons-causing-senescence
- 166
- February 12, 2024
-
Regeneron’s linvoseltamab application accepted for review
- Source: https://www.echnology.com/news/regeneron-linvoseltamab-application-review/
- 91
- February 11, 2024
-
Hemogenyx gets reprieve as FDA lifts clinical hold on AML CAR-T therapy
- Source: drugdu
- 131
- February 10, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.