-
CD3/BCMA/GPRC5D Tri-Specific Antibody MBS314 Approved in Phase I/II Clinical Trial for the Treatment of Multiple Myeloma
- Source: drugdu
- 129
- January 3, 2024
-
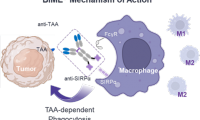
Elpiscience and Astellas Enter into Research Collaboration and License Agreement for Novel Bispecific Macrophage Engager
- Source: drugdu
- 139
- January 2, 2024
-
GFH009 disrupts growth signals and triggers apoptosis in hematologic malignancies
- Source: drugdu
- 127
- January 1, 2024
-
Opdualag licensed for patients with advanced melanoma
- Source: drugdu
- 93
- January 1, 2024
-
HANSIZHUANG Sets Sail in Indonesia Market
- Source: drugdu
- 345
- December 30, 2023
-
Johns Hopkins researchers find minimal regret after gender affirming surgery
- Source: drugdu
- 117
- December 30, 2023
-
Jemincare Announces 6 Approvals of Clinical Trials for its Innovative Drugs
- Source: drugdu
- 97
- December 30, 2023
-
Key Leadership Lessons for Biotech Executives from a Hospital Administrator
- Source: drugdu
- 158
- December 29, 2023
-
Pfizer’s Hospira recall spree bleeds into 2024 with product pulls for multiple hospital drugs in shortage
- Source: drugdu
- 184
- December 29, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.