-
Beijing Municipal Party Secretary Yin Li Met with Novartis Global CEO Wansheim
- Source: drugdu
- 110
- March 29, 2024
-
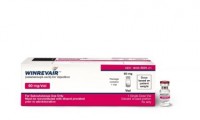
Merck Drug for Heart and Lung Disorder Wins a First-in-Class FDA Approval
- Source: drugdu
- 89
- March 28, 2024
-
$770 Million! AriBio authorizes Marketing Rights for a Drug to Treat Alzheimer’s Disease in China
- Source: drugdu
- 134
- March 28, 2024
-
Novo Nordisk’s Awiqli receives CHMP recommendation to treat diabetes in adults
- Source: drugdu
- 89
- March 27, 2024
-
FDA Nod in Duchenne Helps Wider Swath of Patients With the Rare Muscle Disease
- Source: drugdu
- 163
- March 26, 2024
-
Precision Medicine Startup Mirador Unveils $400M for R&D of New I&I Drugs
- Source: drugdu
- 114
- March 25, 2024
-
FDA Approves First Gene Therapy for Children with Metachromatic Leukodystroph
- Source: drugdu
- 140
- March 20, 2024
-
FDA Approves Tevimbra, BeiGene’s Treatment for Adults with Unresectable or Metastatic Esophageal Squamous Carcinoma
- Source: drugdu
- 124
- March 19, 2024
-
BeiGene’s “tislelizumab” FDA approved
- Source: drugdu
- 114
- March 19, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.