-
Odds of Heart Attack Six Times Higher With Flu Diagnosis
- Source: drugdu
- 407
- April 19, 2023
-
Higher intake of flavonols linked to lower risk of frailty onset in adults
- Source: drugdu
- 351
- April 19, 2023
-
Binocular visual stimulation followed by sleep helps treat common vision problem in children
- Source: drugdu
- 545
- April 19, 2023
-
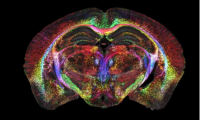
Brain images just got 64 million times sharper
- Source: drugdu
- 551
- April 18, 2023
-
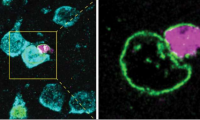
Researchers discover how some brain cells transfer material to neurons in mice
- Source: drugdu
- 368
- April 18, 2023
-
Refined carbs and red meat driving global rise in type 2 diabetes, study says
- Source: drugdu
- 354
- April 18, 2023
-
Practicing and listening to music could prevent working memory decline
- Source: drugdu
- 402
- April 18, 2023
-
Pre- and post-surgical immunotherapy-based treatment significantly improved lung cancer outcomes
- Source: drugdu
- 505
- April 18, 2023
-
Practicing and listening to music could prevent working memory decline
- Source: drugdu
- 550
- April 18, 2023
-
Scientists find mutations, say threat is still low
- Source: drugdu
- 471
- April 17, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.