-
Seasonal Variation in Thyroid Hormone TSH May Lead to Overprescribing
- Source: drugdu
- 503
- April 26, 2023
-
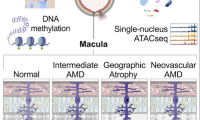
New study of age-related macular degeneration finds causal genes, dispels previous assumptions
- Source: drugdu
- 272
- April 26, 2023
-
Walnuts Linked to Improved Attention, Psychological Maturity in Teens
- Source: drugdu
- 413
- April 26, 2023
-
OTC Narcan will cost ‘less than $50’, company says
- Source: drugdu
- 348
- April 25, 2023
-
BullFrog AI , Sage Group partner to develop oncology assets
- Source: drugdu
- 422
- April 25, 2023
-
Proper nutrition could boost recovery from traumatic brain injury
- Source: drugdu
- 388
- April 25, 2023
-
Drugmakers take sides in Amgen, Regeneron fight over antibody patents
- Source: drugdu
- 453
- April 25, 2023
-
Too much insulin can be as dangerous as too little
- Source: drugdu
- 332
- April 25, 2023
-
How alcohol consumption contributes to chronic pain
- Source: drugdu
- 531
- April 25, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.