-
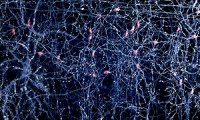
Researchers develop new probes to record neural activity in brain
- Source: medicaldevice-network
- 479
- November 15, 2017
-
A Chinese Province Is Sequencing One Million of Its Residents’ Genomes
- Source: futurism
- 589
- November 10, 2017
-
Innovative heart device is safe and effective, study finds
- Source: Medicalxpress
- 791
- November 2, 2017
-
Apple is testing whether the Apple Watch can detect heart problems
- Source: finance.yahoo
- 828
- September 14, 2017
-
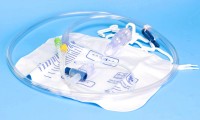
Teleflex’s arterial catheterisation device obtains FDA clearance
- Source: Medicaldevice-network-com
- 453
- July 26, 2017
-
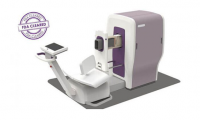
Aspect Imaging’s neonatal MRI device gains FDA clearance
- Source: Medicaldevice
- 503
- July 24, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.