-
Device able to quickly and accurately detect stroke
- Source: Health Data Management
- 725
- April 3, 2018
-
Nine digital health mergers and acquisitions from the first quarter of 2018
- Source: MobiHealthNews
- 495
- April 2, 2018
-
FDA to expand digital health pre-cert program by end of 2018
- Source: Beckers Hospital Review
- 657
- April 2, 2018
-
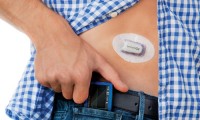
Bigfoot brings in $55M Series B to propel integrated diabetes platform
- Source: MedCityNews
- 803
- March 30, 2018
-
FDA clears new Dexcom CGM that requires no patient calibration earlier than expected
- Source: MedCityNews
- 704
- March 29, 2018
-
Sofinnova Partners Leads SafeHeal’s €6 Million Series a Financing Round
- Source: BusinessWire
- 444
- March 26, 2018
-
Can An mHealth Kit Improve Outcomes in Workers Comp Treatment?
- Source: mHealth Intelligence
- 788
- March 12, 2018
-
Science 37 and Novartis Sign Strategic Alliance to Advance Decentralized Clinical Trials
- Source: Science 37
- 568
- March 12, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.