-
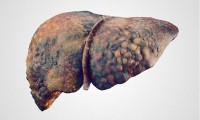
Meta-analysis reports global prevalence of MAFLD of 38.8% in adults
- Source: drugdu
- 409
- August 21, 2023
-
Severe COVID-19 may cause long-term changes to innate immune system
- Source: drugdu
- 382
- August 21, 2023
-
Chimerix’s Drug Candidate Improves Survival for Patients with Incurable Brain Tumor
- Source: drugdu
- 360
- August 21, 2023
-
FDA Approves Ipsen’s Palovarotene for Ultra-Rare Bone Disease
- Source: drugdu
- 315
- August 21, 2023
-
US study finds potential new way of improving immunotherapy for tumours
- Source: drugdu
- 284
- August 21, 2023
-
FDA approves Talvey for heavily pre-treated multiple myeloma
- Source: drugdu
- 371
- August 21, 2023
-
Novo Nordisk spends $1bn to acquire weight loss drug developer Inversago.
- Source: drugdu
- 435
- August 21, 2023
-
GenScript and T-MAXIMUM partner for CAR-T cell therapy development
- Source: drugdu
- 370
- August 21, 2023
-
Amazon Pharmacy to provide automated discounts for insulin in the US
- Source: drugdu
- 379
- August 20, 2023
-
FDA again slams Biocon’s troubled plant in Malaysia, citing 8 manufacturing shortfalls
- Source: drugdu
- 330
- August 20, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.