-
FDA clears Pfizer’s pneumococcal vaccine for infants and children
- Source: drugdu
- 162
- April 30, 2023
-
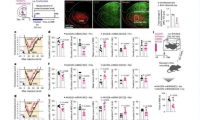
Immunotherapy strongly improves prognosis of babies with leukemia
- Source: drugdu
- 138
- April 28, 2023
-
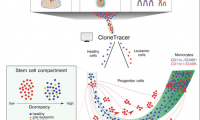
New tool charts differentiation landscape of acute myeloid leukemia
- Source: drugdu
- 124
- April 26, 2023
-
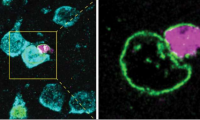
Researchers discover how some brain cells transfer material to neurons in mice
- Source: drugdu
- 148
- April 18, 2023
-
Study unveils neural processes underpinning the re-emergence of consciousness after anesthesia
- Source: drugdu
- 147
- April 14, 2023
-
Spherix
- Source: drugdu
- 134
- April 14, 2023
-
Chiesi Farmaceutici acquires biopharmaceutical firm Amryt Pharma
- Source: drugdu
- 114
- April 14, 2023
-
Scientists develop fastest calcium indicators yet for neural imaging
- Source: drugdu
- 104
- April 6, 2023
-
Cancer drug leaflets for patients in Europe omit important facts
- Source: drugdu
- 201
- April 3, 2023
-
FT
- Source: drugdu
- 519
- March 24, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.