-
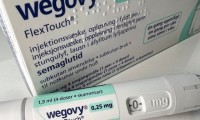
Weight-loss drug firm becomes Europe’s most valuable
- Source: drugdu
- 230
- September 6, 2023
-
Amgen and Horizon Therapeutics settle FTC lawsuit
- Source: drugdu
- 173
- September 6, 2023
-
Delaware’s Marlex Pharmaceuticals recalls 2 batches of heart failure drug after labeling mix-up
- Source: drugdu
- 223
- September 6, 2023
-
Sage Therapeutics Fires 40% Of Staff after FDA Rejected MDD Bid
- Source: drugdu
- 153
- September 5, 2023
-
【EXPERT Q&A】What are the regulations and requirements for exporting medical devices to the European Union?
- Source: drugdu
- 320
- September 5, 2023
-
Zerva acquires Acer Therapeutics to bolster rare disease portfolio
- Source: drugdu
- 105
- September 4, 2023
-
FDA shuns Outlook Therapeutics with wet AMD drug BLA rejection
- Source: drugdu
- 109
- September 1, 2023
-
Reuters
- Source: drugdu
- 165
- August 28, 2023
-
AbbVie achieves win for migraine prevention drug Aquipta in Europe
- Source: drugdu
- 120
- August 22, 2023
-
A Comprehensive Guide
- Source: drugdu
- 355
- August 18, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.