-
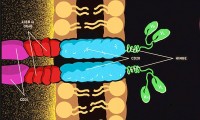
Tmunity nabs $100M for next-gen T-cell immunotherapies
- Source: fiercebiotech
- 505
- January 26, 2018
-
Janssen buys into Legend’s CART therapy
- Source: Pharmatimes
- 412
- December 26, 2017
-
Gilead Sciences to Acquire Cell Design Labs for $567 Million
- Source: qc.nasdaq
- 427
- December 11, 2017
-
Kite’s Yescarta™ (Axicabtagene Ciloleucel) Becomes First CAR T Therapy Approved by the FDA
- Source: Businesswire
- 679
- October 20, 2017
-
FDA Okays Another CAR T-Cell Therapy
- Source: medpagetoday
- 744
- October 19, 2017
-
Over $440 million! Jiahe Biologics’ new dual-antibody drug goes overseas
- Source: drugdu
- 66
- October 11, 2024
-
Fosun Pharma achieves 100% control
- Source: drugdu
- 71
- September 27, 2024
-
Gilead writes $2.4B off Trodelvy as CEO underscores ‘time of focused execution’
- Source: drugdu
- 126
- April 30, 2024
-
Legend Biotech’s “CARVYKTI®” was approved for second-line indications in the United States
- Source: drugdu
- 107
- April 8, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.