-
Novartis Phase III study demonstrates adjuvant Tafinlar® + Mekinist® reduced the risk of disease recurrence by 53%
- Source: Novartis
- 827
- September 13, 2017
-
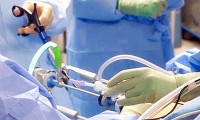
Stent Tek Closes Series A Investment Round for its ePATH AVF Device, Aiming to Improve Vascular Access for Haemodialysis
- Source: prnewswire
- 587
- September 8, 2017
-
Apollo Endosurgery gets CE-Mark for managed weight loss system
- Source: medicaldevice-network
- 886
- September 4, 2017
-
Stent Tek Closes Series A Investment Round for its ePATH AVF Device, Aiming to Improve Vascular Access for Hemodialysis
- Source: infomeddnews
- 406
- September 1, 2017
-
MicroPort Orthopedics launches new acetabular cup system
- Source: medicaldevice-network
- 472
- August 31, 2017
-
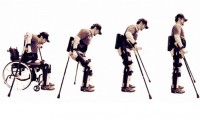
NIH develops robotic exoskeleton to treat crouch gait in children
- Source: medicaldevice-network
- 657
- August 30, 2017
-
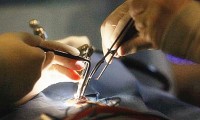
New tech “lights up” nerves to identify, reduce accidental damage
- Source: massdevice
- 483
- August 21, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.