-
Novartis Scores Phase III Chronic Hives Wins as Sanofi Challenges
- Source: drugdu
- 209
- August 11, 2023
-
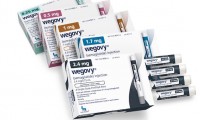
Wegovy shown to reduce risk of heart attack, stroke in major cardiovascular trial
- Source: drugdu
- 125
- August 11, 2023
-
Astellas invests $50m to support advancement of Poseida’s cancer cell therapy
- Source: drugdu
- 111
- August 11, 2023
-
Celmatix and Aché Laboratórios partner to tackle unmet need for PCOS drugs
- Source: drugdu
- 114
- August 11, 2023
-
Guardant, Illumina agree to end litigation, extend commercial partnership
- Source: drugdu
- 178
- August 10, 2023
-
Dendritic cells found to kill CD47-deficient T cells
- Source: drugdu
- 113
- August 10, 2023
-
Novavax Posts Surprise Q2 Profit, Hinges Hopes on Updated COVID Shot
- Source: drugdu
- 244
- August 10, 2023
-
Novo Nordisk posts ‘best-case scenario’ cardio outcomes data for star obesity drug Wegovy
- Source: drugdu
- 136
- August 10, 2023
-
J&J signs Stelara biosimilar settlement deal with Formycon and Fresenius
- Source: drugdu
- 186
- August 10, 2023
-
Merck and Ginkgo Bioworks enter biologic manufacturing collaboration worth up to $490m
- Source: drugdu
- 103
- August 10, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.