-
Landmark Clinical Approval for YOLT-201 Obtained by the NMPA
- Source: drugdu
- 89
- March 10, 2024
-
CPhI Japan 2024
- Source: drugdu
- 174
- March 8, 2024
-
Simple Measurement Predicts Risk of Rapid Progression of Chronic Kidney Disease
- Source: drugdu
- 100
- February 29, 2024
-
Inclusion of noncommunicable disease care in response to humanitarian emergencies will help save more lives
- Source: drugdu
- 121
- February 29, 2024
-
TransThera initiates IND-Enabling studies for TT-02332, a novel, highly potent and CNS-penetrating NLRP3 inhibitor
- Source: drugdu
- 140
- February 28, 2024
-
Study Finds Parkinson Disease Drug Nearly Matches Wegovy in Weight Loss Effects
- Source: drugdu
- 183
- February 26, 2024
-
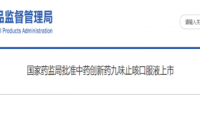
Zhuohe Pharmaceutical’s “Category 1.1 innovative traditional Chinese medicine” was approved for marketing
- Source: drugdu
- 148
- February 26, 2024
-
Innovent and ImmVirX Enter into Clinical Trial Collaboration to Investigate the Combination of IVX037 and Sintilimab in Difficult-to-treat Cancers
- Source: drugdu
- 157
- February 26, 2024
-
FDA grants orphan drug status to Ionis’ olezarsen for FCS treatment
- Source: drugdu
- 132
- February 19, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.