-
First Patient Dosed in Clinical Trial of YOLT-101 for the Treatment of FH
- Source: drugdu
- 133
- April 4, 2024
-
GlobalData
- Source: drugdu
- 83
- April 3, 2024
-
FDA Hands Expanded Indication to Gilead’s Vemlidy for Chronic HBV Infection in Pediatric Patients
- Source: drugdu
- 88
- April 2, 2024
-
J&J reportedly eyeing Shockwave Medical for takeover
- Source: drugdu
- 112
- April 2, 2024
-
Hengrui’s Clinical Application for New Drug Indicated for Psoriasis Approved
- Source: drugdu
- 90
- April 2, 2024
-
CPhI North America 2024
- Source: drugdu
- 163
- March 29, 2024
-
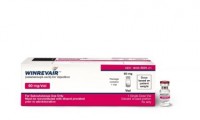
Merck Drug for Heart and Lung Disorder Wins a First-in-Class FDA Approval
- Source: drugdu
- 91
- March 28, 2024
-
Another Chiatai Tianqing’s contrast agent, iopromide injection, was approved for marketing
- Source: drugdu
- 142
- March 28, 2024
-
CPhI South East Asia 2024
- Source: drugdu
- 178
- March 26, 2024
-
Four international teams awarded £4.7m to investigate heart and circulatory diseases
- Source: drugdu
- 107
- March 26, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.