-
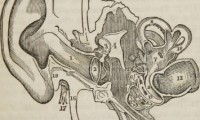
Eli Lilly Sounds Off as Early Data Show How Gene Therapy Can Restore Hearing
- Source: drugdu
- 86
- January 26, 2024
-
Bayer CEO Bill Anderson makes his mark with major restructuring, ‘significant’ job cuts
- Source: drugdu
- 189
- January 20, 2024
-
Pfizer’s eye for China ADC deals; Astellas’ gastric cancer rejection
- Source: drugdu
- 141
- January 17, 2024
-
Manufacturing Issues Block Astellas From a First-in-Class Cancer Drug Approval
- Source: drugdu
- 226
- January 16, 2024
-
S&P Global Releases Healthcare Industry Outlook for 2024
- Source: drugdu
- 184
- January 15, 2024
-
3M Health receives grant to develop wound treatment solutions in US
- Source: drugdu
- 178
- January 15, 2024
-
Pfizer CEO promises ‘year of execution’ after recent struggles, Seagen buyout
- Source: drugdu
- 94
- January 13, 2024
-
What Was the Most-Repeated Word in CVS Health CEO’s JPM Presentation?
- Source: drugdu
- 122
- January 11, 2024
-
At JPM, Top GSK Cancer Executive Talks Drug Targets and Oncology Strategy
- Source: drugdu
- 121
- January 11, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.