-
FDA clears Pfizer’s pneumococcal vaccine for infants and children
- Source: drugdu
- 161
- April 30, 2023
-
Exploring Doggybone ™ DNA Technology
- Source: drugdu
- 117
- April 30, 2023
-
UK monkeypox vaccine rollout to wind down
- Source: drugdu
- 146
- April 28, 2023
-
Pfizer, BioNTech enlist Marvel’s Avengers in latest COVID-19 vaccine booster push
- Source: drugdu
- 241
- April 28, 2023
-
Adding new vaccine type to leading immunotherapy dramatically reduced melanoma recurrence
- Source: drugdu
- 230
- April 17, 2023
-
Moderna cancer vaccine takes positive step
- Source: drugdu
- 112
- April 12, 2023
-
Pfizer and Moderna’s COVID-19 vaccines offer lasting protection
- Source: drugdu
- 175
- April 12, 2023
-
Sanofi, BARDA break ground on another flu vaccine plant at Pennsylvania campus
- Source: drugdu
- 146
- April 7, 2023
-
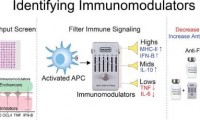
Immunomodulators boost vaccine response and reduce inflammation, finds study
- Source: drugdu
- 150
- April 6, 2023
-
mRNA vaccine beats infection for key defense against COVID-19, Stanford Medicine scientists find
- Source: drugdu
- 143
- March 31, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.