-
New Male Contraceptive Pill With No Side Effects
- Source: Ddu
- 1,213
- June 7, 2018
-
Lynparza and Zytiga Combo Effective Against Prostate Cancer
- Source: Ddu
- 758
- June 6, 2018
-
Pneumonia Detection mHealth Wearable
- Source: mHealth Intelligence
- 844
- June 5, 2018
-
Parachute Health Received $9.5 Million Funding for ePrescribing Platform
- Source: Business Insider
- 501
- June 5, 2018
-
Data From Grail Shows Early Detection Of Cancer
- Source: FierceBiotech
- 518
- June 5, 2018
-
NIPT to Detect Chromosomal Disorders in Unborn Babies
- Source: Ddu
- 560
- June 5, 2018
-
Ibuprofen Affects Daughters’ Fertility In Future
- Source: Ddu
- 606
- June 4, 2018
-
Niti Aayog Planning to Improve Indian Medical Devices Sector
- Source: Ddu
- 839
- May 31, 2018
-
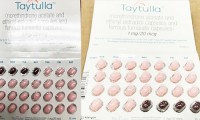
Packaging Error Prompts Allergan to Recall Sample Packs of Taytulla Contraceptive Pills
- Source: Ddu
- 871
- May 30, 2018
-
Market for Mobile Health Technology To Exceed USD 28 Billion In 2018
- Source: Ddu
- 906
- May 30, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.